The younger the child, the more reluctant the parents.
Just three in 10 parents of children under 5 say they’ll get their child vaccinated right away once a COVID-19 vaccine is approved for their age group, according to new survey findings published this month by KFF, a healthcare think tank. While that’s lower than the rate for older children, it’s still up from one in five parents in July.
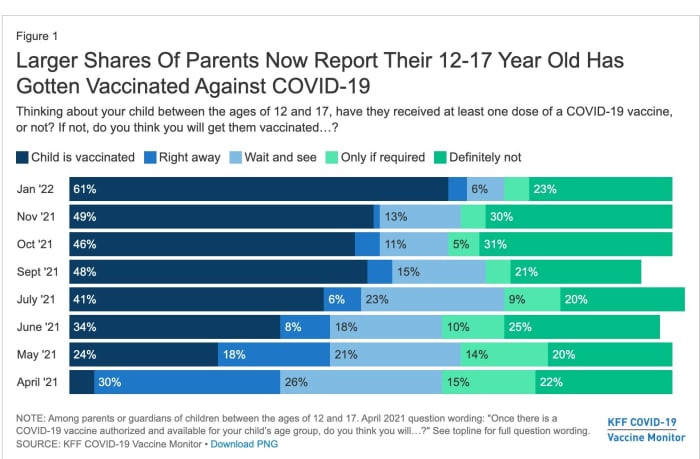
Parents are more willing to get their older children vaccinated: Just 33% of parents of 5- to 11-year-olds say their child has had at least one COVID-19 shot, though that’s up from 16% in November, while 61% of parents of 12- to 17-year-olds said their child had received at least one shot, up from just under 50% in November.
“‘Half of parents report being worried about their child becoming seriously sick from the coronavirus.’”
On Tuesday, Pfizer PFE, +1.49% and BioNTech BNTX, +0.31% asked federal regulators to authorize Pfizer’s COVID-19 vaccine for children as young as 6 months. Those first shots could come by the end of February. Pfizer’s vaccine is currently available for 5- to 11-year-olds, in a dosage one-third of what people 12 and older receive.
“Many parents of school-aged children say their child has experienced some disruption in their schooling during January, including having to quarantine, having the school shut down or move to online learning, or parents choosing to keep their child home due to COVID-19 concerns,” KFF said.
And yet many reluctant parents say they’re concerned about the safety of the coronavirus vaccines. “Half of parents report being worried about their child becoming seriously sick from the coronavirus, including substantially higher shares among parents who are Black or Hispanic and those with lower incomes,” the report added.
Scientists have conducted clinical trials of the vaccines with thousands of children and found no serious safety concerns, according to the Centers for Disease Control and Prevention. “COVID-19 vaccines are being monitored for safety with the most comprehensive and intense safety monitoring program in U.S. history,” the agency says.
Pfizer chief executive Albert Bourla noted Tuesday that hospitalizations of children under 5 infected with the virus had soared.
“Ultimately, we believe that three doses of the vaccine will be needed for children 6 months through 4 years of age to achieve high levels of protection against current and potential future variants,” he said.
